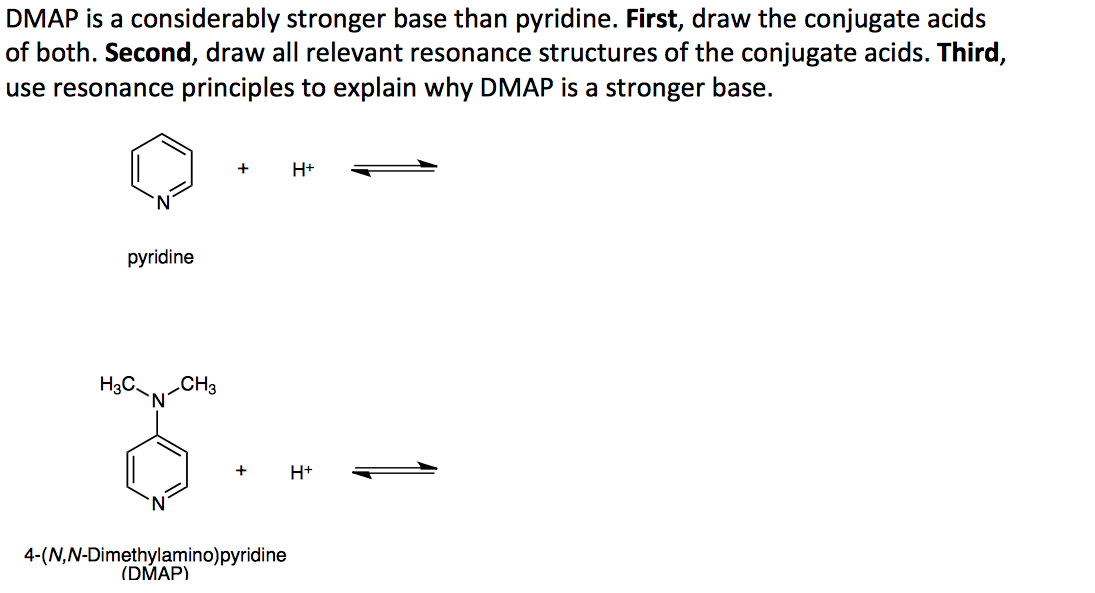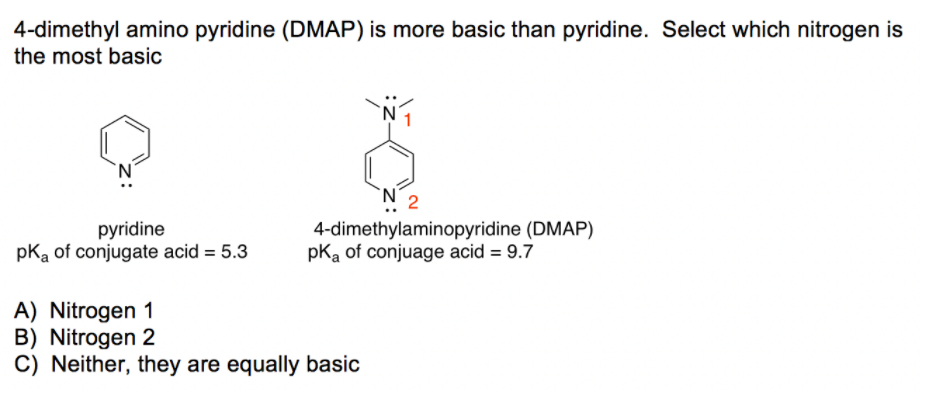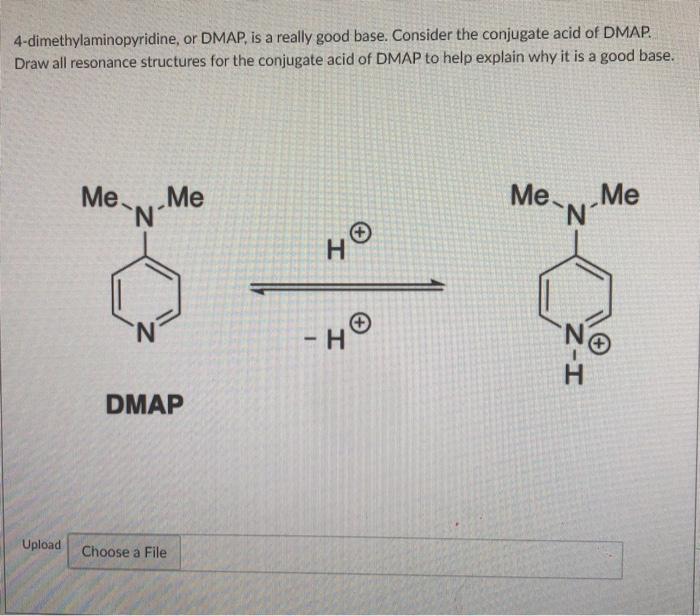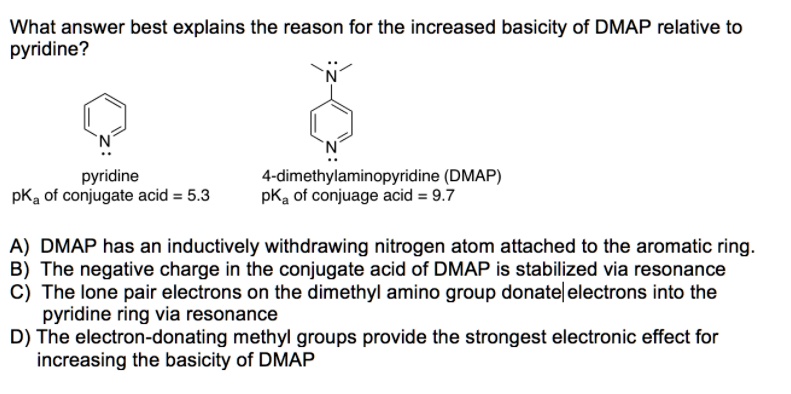
SOLVED: What answer best explains the reason for the increased basicity of DMAP relative to pyridine? pyridine 4-dimethylaminopyridine (DMAP) pKa of conjuage acid = 9.7 pKa Of conjugate acid = 5.3 A)

4-(N,N-Dimethylamino)pyridine Hydrochloride as a Recyclable Catalyst for Acylation of Inert Alcohols: Substrate Scope and Reaction Mechanism | Organic Letters
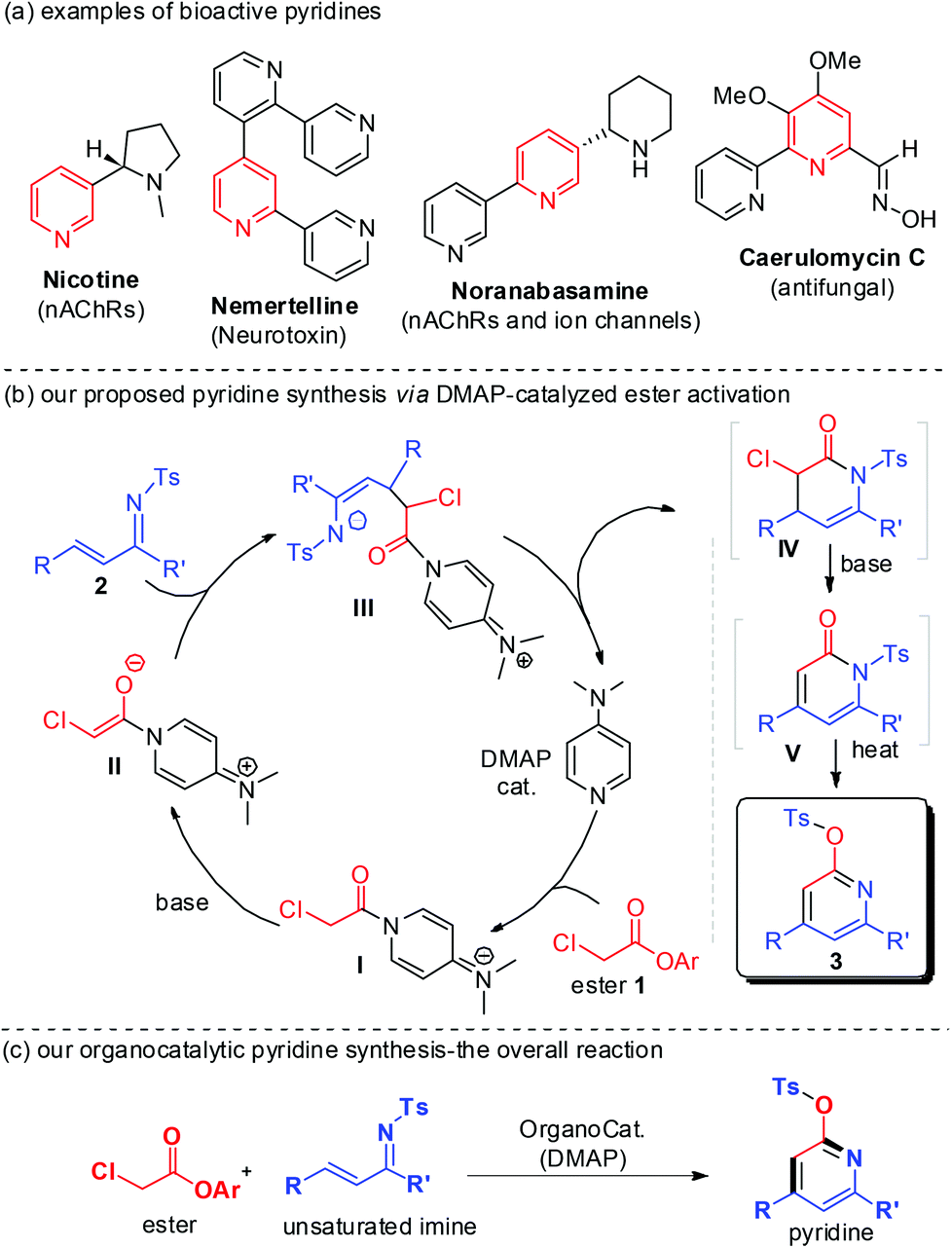
Access to pyridines via DMAP-catalyzed activation of α-chloro acetic ester to react with unsaturated imines - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C3QO00045A

Fluorous 4‐N,N‐Dimethylaminopyridine (DMAP) Salts as Simple Recyclable Acylation Catalysts - Vuluga - 2010 - Chemistry – A European Journal - Wiley Online Library

Widely Useful DMAP-Catalyzed Esterification under Auxiliary Base- and Solvent-Free Conditions | Journal of the American Chemical Society

Steric Effects in the Uncatalyzed and DMAP‐Catalyzed Acylation of Alcohols—Quantifying the Window of Opportunity in Kinetic Resolution Experiments - Fischer - 2006 - Chemistry – A European Journal - Wiley Online Library