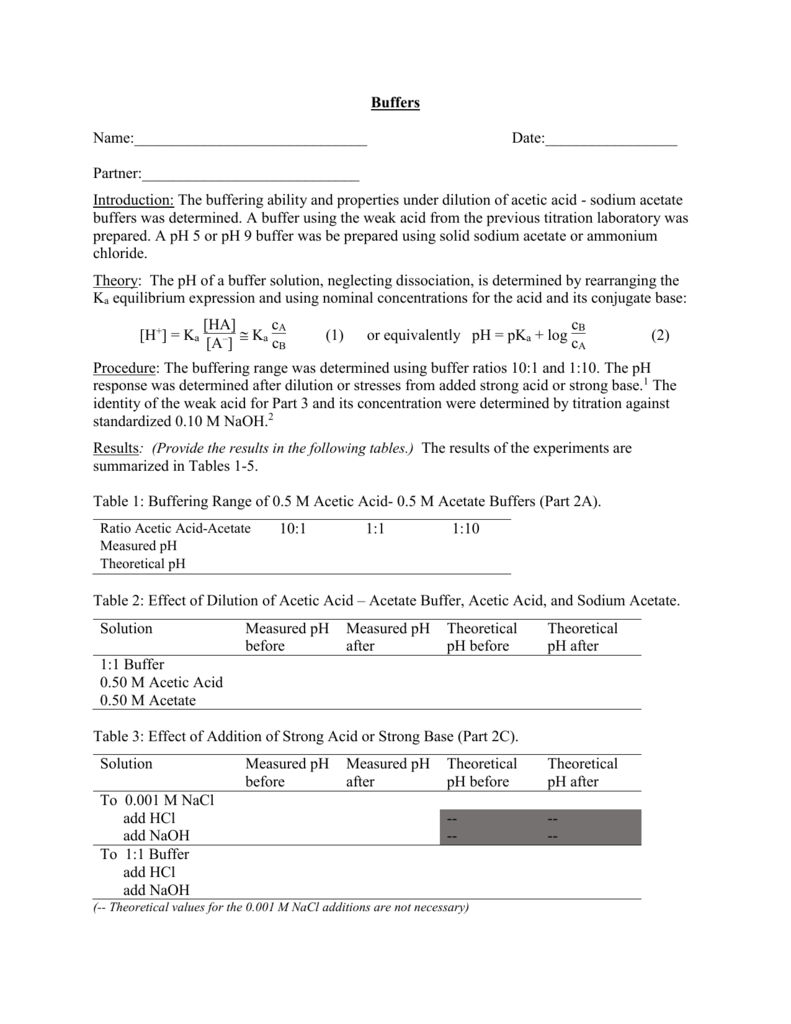
SCH 4 U 1. What are buffers? Buffers are mixtures of conjugate acid- base pairs that allow a solution to resist changes in pH when acids and/or bases. - ppt download

1 Chapter 10 Acids and Bases 10.9 Buffers. 2 When an acid or base is added to water, the pH changes drastically. A buffer solution resists a change in. - ppt download

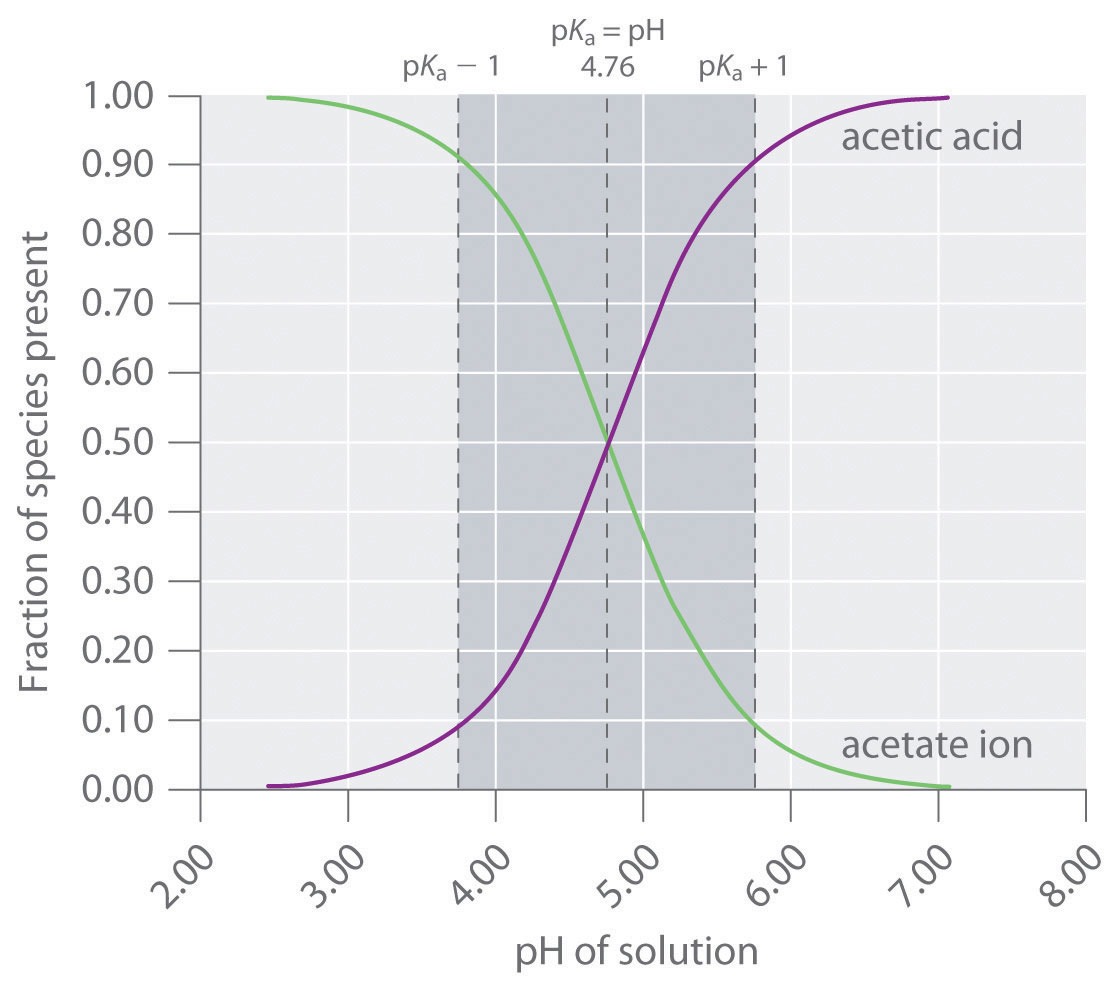
50 mL of 0.1 M solution of sodium acetate and 50 mL of 0.01 M acetic acid are mixed. The pKa of acetic acid is 4.76. The pH of the buffer solution is:
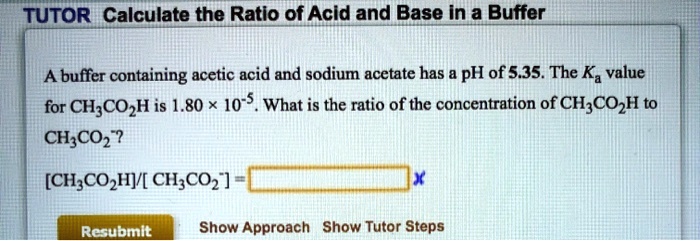
SOLVED: TUTOR Calculate the Ratio of Acid and Base In a Buffer A buffer containing acetic acid and sodium acetate has pH of 5.35. The Ka value for CH;COzH is 1.80 x

What is the pH of buffer solution containing 0.17 M acetic acid and 0.36 M sodium acetate? - YouTube
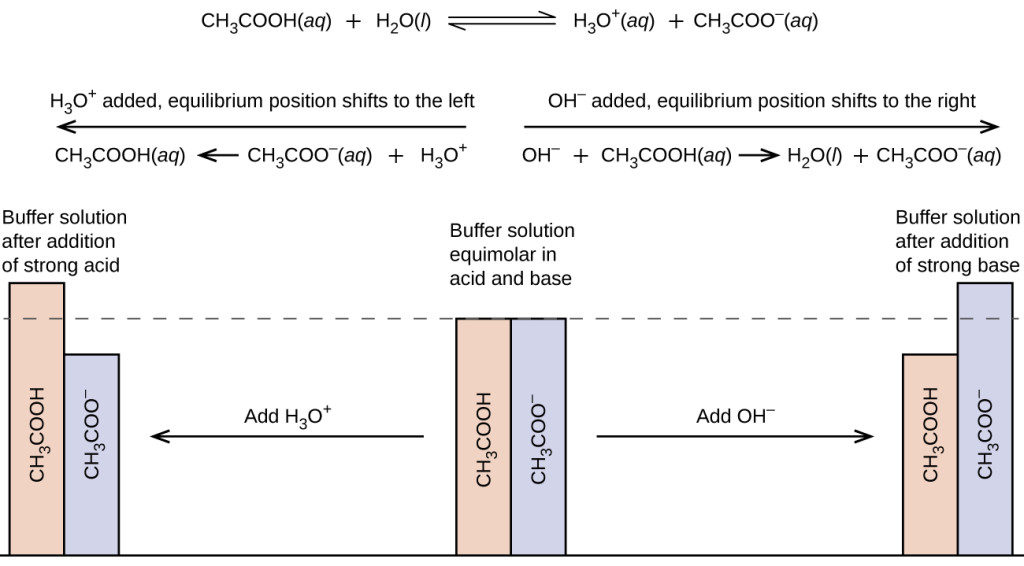
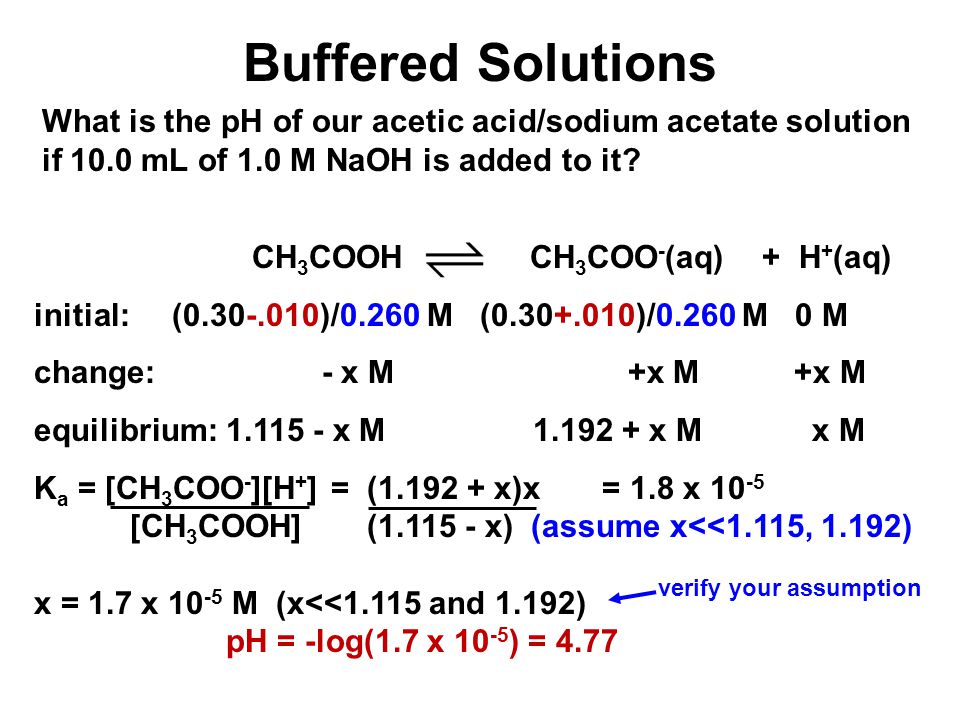
Prove the buffer action of acetic acid and sodium acetate by the addition of 0.01 mol of solid sodium hydroxide. - Sarthaks eConnect | Largest Online Education Community

In a mixture of acetic acid and sodium acetate, the ratio of concentrations of the salt to the acid is increased ten times. Then the pH of the solution: